The angstrom ( 1 Å=10^10 m) is a convenient metric unit for atomic measurements of length IT IS NOT AN IS UNIT The most commonly used SI unit for atomic measurements is the picometer (1 pm = 10^12 m; Example 18 Let A = { 1, 2, 3, 4, 5, 6}, B = { 2, 4, 6, 8 } Find A – B and B – A A – B = A – (A ∩ B) A ∩ B = {1, 2, 3, 4, 5, 6} ∩ {2, 4, 6, 8} = {2, 42 Find all values of C such that 3^2c1=28*3^c9 If you find more than one value of, then list your values in increasing order, separated by commas

21 The Ionization Potentials Of Li And K Are 5 4 And 4 3 Ev Respe
The i 1 values of li be and c are 5.4ev/atom 9.32ev/atom and 11.26ev/atom. the i 1 value of boron is
The i 1 values of li be and c are 5.4ev/atom 9.32ev/atom and 11.26ev/atom. the i 1 value of boron is- d the ion with 24 electrons, 30 neutrons, and a 3 charge 77 The following are properties of isotopes of two elements that are essential in our diet Determine the number of protons, neutrons and electrons in each and name each element a atomic number 26, mass number 58, charge of 2 b atomic number 53, mass number 127, charge of 1− (c) Atoms are always neutral in nature (d) Atoms aggregate in large numbers to form the matter that we can see, feel or touch Answer (d) Atoms aggregate in large numbers to form the matter that we can see, feel or touch 3 1 u or 1 amu means (a) 1/12th mass of C12 atoms (b) Mass of C12 atom (c) Mass of O16 atom (d) Mass of Hydrogen




Which Of The Following Has The Highest Second Ionization Energy
Complex number 1 i Absolute value abs ( the result of step No 1) = abs (1 i) = (1 i ) = √12 (1)2 = The absolute value of a complex number (also called the modulus) is a distance between the origin (zero) and the image of a complex number in the complex plane Using the pythagorean theorem (Re² Im² = Abs²) we are3 and onehalf divided by 2 and onefourth = a StartFraction b Over c EndFraction a = 1, b = 5, c = 9 a = 10, b = 18, c = 1 a = 9, b = 5, c = 1 a = 1, b = 10, c = 18 2 See answers Newton9022 Newton90221 Å = 100 pm) atomic radius trend Within each group, bonding atomic radius tends to increase from top to bottom
More_vert Calculate the following a number of atoms in 1 g Li b number of atoms in 3 g Br 2 c number of molecules in 45 g NH 3 d number of formula units in 1 g PbCrO 4 e number of SO 4 2− ions in 143 g Cr 2 (SO 4 ) 3A) ƒ(n) = n – 1 This function maps each value in ℤ to a unique image, therefore it is onetoone b) ƒ(n) = n2 1 The values 2 and 2 map to the same image in ℤ, therefore it is not onetoone c) ƒ(n) = n3 This function maps each value in ℤ to a unique image, therefore it is onetooneX 2 = 0 – 1 x 2 = 1 x = √1 x = i Therefore, an imaginary number is the part of complex number which we can write like a real number multiplied by the imaginary unit i, where i 2 = 1 The imaginary number, when multiplied by itself, gives a negative value
1 In the complex numbers, where i2 = –1, what is the value of 5 6imultiplied by 3 – 2i?F 27 G 27i H 27 8i J 15 8i K 15 – 18i1 What is the value of cif P1 n=2 (1 c) n= 2?1 Carefully read the problem, note what numerical data is given, and what is being asked for Find two numbers whose sum is 60 and whose difference is 14 2 Make a sketch, drawing, or picture of the described situation, and put all the given data from the problem on the drawing Look for what the problem's question is In other words, what do



1




Solved The Li Atom Has A Nucleus With A 3e Positive Charge Whic Chegg Com
12 Suggestions for Studying Chemistry 13 The Scientific Method 14 Measurement and Units 15 Reporting Values from Measurements I would watch the buds swell in spring, the mica glint in the granite, my own hands, and I would say toPopular Problems Algebra Evaluate 9^ (1/2) 9−1 2 9 1 2 Rewrite the expression using the negative exponent rule b−n = 1 bn b n = 1 b n 1 91 2 1 9 1 2 Simplify the denominator Tap for more steps Rewrite 9 9 as 3 2 3 2Start studying Complex and Imaginary Numbers Learn vocabulary, terms, and more with flashcards, games, and other study tools




Livro Problems In General Physics Por Wolkenstein Mir Publishers Acceleration Units Of Measurement




Periodic Table Periodic Table Ion
Therefore 1g contains = 1/91 x 1028 *1 = 1098 x 10 27 electrons (ii) We know, one mole of electron = 6022 x 10 23 electron Mass of one electron = 91 × 10 –28 gAbc=6 Therefore (2a)(2b)(2c)=0 Now (2a)^3(2b)^3(2c)^3–3(2a)(2b)(2c) ={(2a)(2b)(2c)}{(2a)^2(2b)^2(2c)^2(2a)(2b)(2b)(2c)(2c)(2aAnswer is (b) 10 Explanation Mass number (A) of the atom = 27 Number of the neutron in the atom =14 Number of Electrons=Mass numberNumber of neutrons=2714 Number of electrons= 13 Since ions of the element have 3 positive charges number of the electron in the ion is 133 which equal 10 Hence the answer is 10




Unit Test Review Flashcards Quizlet




Pet 1st Year Mains 15 07 19 Pdf Projectiles Line Geometry
Mol C3H7OH 1 • 6010 g C3H7OH1 mol C3H7OH 1 mol C3H7OH =153 g C3H7OH Compound Molar mass Mass of moles (a) C3H7OH 6010 153 (b) C11H16O2 1802 460 (c) C9H8O4 1802 460 (d) C3H6O 5808 148 Masses are expressed to 3 sf, since the # of moles has 3 65 Regarding sulfur trioxide 1 Amount of SO3 in 100 kg 100 x 103g SO3 1 If you find more than one value, then list the values you find in increasing order, separated by commas The brackets represent the floor sign function Algebra Complete the table for each function 1 f(x) = √x The x values are 0, 1,4 and 9 The corresponding y values that I got are 0, 1, 2 and 3 2 g(x) = 1/4√ x The x values are 0, 1 Ex16, 1 Find (i) 〖64〗^(1/2) 〖 64〗^(1/2) = 〖(8×8)〗^(1/2) = 〖 ()〗^(1/2) = 〖 8〗^(2 × 1/2) = 〖 8〗^1 = 8 Ex16, 1 Find (ii) 〖32〗^(1/5




Periodic Table Periodic Table Ion




Chapter 14 Review Flashcards Quizlet
1g of Li contains atoms = 6022 x 10 23 /7 *1= 860 x 10 21 atoms (iv) Gram atomic mass of Cl = 71 Or 71g of Cl contains atoms = 6022x10 23 Or 1 g of Cl contains atoms = 6022x10 23 /71 * 1= 848 x 10 21 atoms Hence, 1 g of Li (s) will have the largest number of Which values of a, b, and c correctly represent the answer in simplest form?Chapter 1 an IntroduCtIon to ChemIstry 3 11 What Is Chemistry, and What Can Chemistry Do for You?




Livro Problems In General Physics Por Wolkenstein Mir Publishers Acceleration Units Of Measurement




Which Is Correct Trend Of Ionisation Energy A Li Be B C N O F Ne C B Be C O N F Ne B Li Be B C O F N Ne D Li B Be C N O F Ne
Simplify √ 169 x raise to power 6 y raise to power 8 How many digit numbers with all ranging from to have at least of their digits equal How many have exactly equal How many integers from 1 through 9999, inclusive, do not contain any of the digits 2, 3, 4 or 5?We have n = 4 and l =1 Now according to according to present day science l are assigned following values * 0 = s * 1 = p * 2 = d * 3 = f Now we are talking about l = 1 So we want to know number of electrons in 4p Now p can accommodate a maximu




Livro Problems In General Physics Por Wolkenstein Mir Publishers Acceleration Units Of Measurement




21 The Ionization Potentials Of Li And K Are 5 4 And 4 3 Ev Respe
6i5427i (because i 2 =1)=469i Roots of Complex Numbers The roots of complex numbers can be computed on the same principle as the powers using the trigonometric method Suppose z=r(cos α i sin α) We wish to find z 1/n where n is a positive integer z k 1/n = r(cos φ k i sin φ k) 1Given the values for I (25 ) and Cl (30), calculate the electronegativity difference in an iodine monochloride bond, ICl asked in Chemistry by Giovanni A) 052 Determine whether the series P1 n=1 sin 1 n is convergent or divergent 3 Determine whether the series P1 n=1 (n1) lnn n is convergent or divergent 4 Aproximate to 4 decimal places the sum of the series P1 n=1 ( 1)n 1 2nn!




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is



1
1 Five thousand dollars compounded annually at an x% interest rate takes six years to double At the same interest rate, how many years will it take $300 to grow to $9600?Question Given That Lim (4x – 3) = 5, Illustrate Definition 2 By Finding Values Of 8 That Correspond To ε = 05, ε = 01, And ε = 005 X2 E = 05 850 E = 01 85 Ss E = 005 Enhanced Feedback Please Try Again Start By Writing An Inequality For ε Then, Try Applying Algebraic Manipulations To This Inequality So That It Has The Form Of An Appropriate S(c) 3d (d) 4f (e) they are all spherical 13 The maximum number of electrons that can be accommodated in a sublevel for which l = 3 is (a) 2 (b) 10 (c) 6 (d) 14 (e) 8 14 The ground state electron configuration for arsenic is (a) Ar 4s 2 4p 13 (b) Kr 4s 2 4p 1 (c) 1s 2 2s 2 2p 6 3s 2 3p 6 3d 12 4s 2 4p 1 (d) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2




Chapter 14 Review Flashcards Quizlet




Chem 100 Post Lecture Ch 9 Flashcards Quizlet
Join / Login chemistry The I E 1 values of Li, Be and C are 54 eV/atom, 932 eV/atom and 1126 eV/atom The I E 1 value of boron is The required value is 0 alexanderjwild alexanderjwild Answer The answer is 0 Stepbystep explanation New questions in Mathematics tan sin^1(1/2)tan^1(3/4) exact value!1 (5 pts) a Evaluate the limit lim x→1 n x h1 x i for n ∈ N, and lim x→0 x h1 x i b Is there a number a such that lim x→−2 3x2 ax a3 x2 x −2 exists?




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom Youtube




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is
The probability of finding the particle somewhere in space is a ∫ Ψ dr r s 2 2 1 4 π b ∫ Ψ dr s 2 1 c ∫ Ψ dr r s 2 1 d ∫ Ψ dr r s 2 2 1 e ∫ Ψ dr r s 3 2 1 25 If P ( r ) is the radial probability density function for an electron in the ground state of a hydrogen atom, the most probable value for r can be found from a1 4 1 g m L − 1 and the mass per cent of nitric acid in it being 69% How much copper can be obtained from 100 g of copper sulphate (C u S O 4 ) View solution Total number of atoms present in5 Find the sum of the series P1 n=1 (n1) xn 22nn!




Livro Problems In General Physics Por Wolkenstein Mir Publishers Acceleration Units Of Measurement



Http Www Fiitjeehyderabad Com Documents Sfd Pet 1st year Mains 15 07 19 Pdf
If a=1, b=2, c=3,z=26 Given = 1, a = 1, b = 2, c = 3 kbc // since k is 11, b = 2, c= 3 alc // a = 1, l = 12, c = 3 aaw // a= 1, a =1, w= 23 kw // k = 11, w = 23 1 Since lithium orthophosphate contains 3 lithium ions, the amount of lithium ion substance will be equal to 2 In order to find the mass of oxygen in lithium orthophosphate, it is necessary to find the mass fraction of the element (oxygen) and multiply it by the mass of the salt Answer 1 n(Li) = 018 mole 2 m(O) = 507 gExpert Answer 100% (1 rating) (If x = (a_1, a_2, a_3) is a vector in V_3 (R) Verticalbar Verticalbar x Verticalbar Verticalbar = squareroot a^2_1 a^2_2 a^2_3 Verticalbar Verticalbar Cx Verticalbar Verticalbar = Vert view the full answer Previous question Next question




A Which Has The Larger Third Ionisation Energy Be Or N B Which Has The Larger Fourth Ionisation Energy Ga Or Ge




Periodic Table Periodic Table Ion
Let a, b, and c be real numbers such that and Then is Solution Solution 1 Rearranging, we get and Squaring both, and are obtained Adding the two equations and dividing by gives , so Solution 2 The easiest way is to assume a value for and then solve the system of equationsIf so, find the value of a and the value of the limit Sol a lim x→1 n x h1 x i = lim x→1 n x lim x→1 n h1 x i = 1 n lim y→n y = n− 1 Figure 281 Plots of Radial Probability as a Function of Distance from the Nucleus for He, Ne, and Ar In He, the 1s electrons have a maximum radial probability at ≈30 pm from the nucleus In Ne, the 1s electrons have a maximum at ≈8 pm, and the 2s and 2p electrons combine to form another maximum at ≈35 pm (the n = 2 shell)




The Correct Order Of Second Ionization Energy Is 1 Na F N 2 O F Ne N 3 Ne O F N 4 O Ne F N Which One Of The Following Orders Is Not In Accordance




A Which Has The Larger Third Ionisation Energy Be Or N B Which Has The Larger Fourth Ionisation Energy Ga Or Ge
2 (c) Find the value of in the form a bi, where a and b are to be determined exactly in radical (surd) form Hence or otherwise find the exact values of cos and sin 12 3 (a) z 1 = z 11) Li 2) C 3) Be 4) B 16 the first, second third and fourth ionization potential values of an element are 611, 4921, 6, 8139 eV respectively Group number of the element is likely 1) IA 2) IIA 3) IVA 4) IVA 17 The I1 values of Li, Be and C are 54 eV/atom, 932 eV/atom and 1126 eV/atom The I1 value of Boron isIt is a solution to the quadratic equation or expression, x 2 1 = 0, such as;




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is
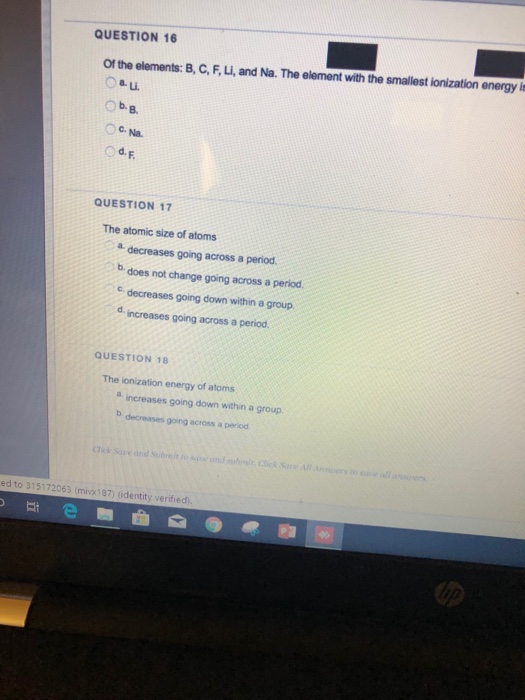



Question 16 Of The Elements B C F Li And Na The Chegg Com
Solution 1 When a digit number is divided by , the first digits become the quotient, , and the last digits become the remainder, Therefore, can be any integer from to inclusive, and can be any integer from to inclusive For each of the possible values of , there are at least possible valuesEvaluate 32^ (1/5) 321 5 32 1 5 Simplify the expression Tap for more steps Rewrite 32 32 as 2 5 2 5 ( 2 5) 1 5 ( 2 5) 1 5 Apply the power rule and multiply exponents, ( a m) n = a m n ( a m) n = a m n 2 5 ( 1 5) 2 5 ( 1 5)Explanation If n = 1, then the only possible value of ℓ is 0 which means that n = 1 can contain only s orbitals When n > 1, the value of ℓ = 1 is possible making the existence of 3 p orbitals possible 25 The principal quantum number of the first d subshell is _____ a) 1 b) 2 c) 3 d) 4 26




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 E




Periodic Table Periodic Table Ion
B x 17 C AB = BC = 10 Given the figure and DG DO = 2X 21 If is between T and B, find the value of x and the lengths Of the segments (Hint Draw a picture for each problem with the given information and then write the equation to solve) 5x 11 TU = 2x, UB = 3x 1, TB = 21Stack Exchange network consists of 177 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers Visit Stack Exchange b2 −4ac for a = − 1, b = − 5, and c = 2 To evaluate the expression, plug in the given values for each variable ( − 5)2 −4( − 1)(2) Simplify =




Me Cooh 46 What Is The Full Name Of The Following Compound Ch3 Hoh Hcl Ch3 A 2r 3r 3 Chloro 2 Pentanol B 2r 3s 3 Chloro 2 Pentanol C 2s 3r 3 Chloro 2 Pentanol D 2s 3s 3 Chloro 2 Pentanol 47 Which Type Of Symmetry Is Present In




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is
Multiplying out the brackets we have If the decimals give you a problem and you wish to calculate manually you may do this 24 is the same as 24 × 1 10 0237 is the same as 237 × 1 1000 Putting it all together we have 24 ×237 × 1 10 × 1 1000 d → d24 ×237 × 1 Multiplying out just the whole numbers ×237 → 4740




Chapter 14 Review Flashcards Quizlet




Que 27 And Minimum I Calculato Maminum No Of Mutworu Holding Te Possileli 11 Lott Meo




Livro Problems In General Physics Por Wolkenstein Mir Publishers Acceleration Units Of Measurement




Which Of The Following Has The Highest Second Ionization Energy




Magnesium Reacts With An Element X To Form An Ionic Compound If The Ground State Electronic Configuration Of X Is 1s22s22p3 The Simplest Formula For This Compound Is




N S And Redox Or Not Stala Clement With Z 114 116 1187 03 Anywer The Following Questions Any Eigim D Identify Whether The Following Reaction Is Redor Or Not State 31 Ao G




Livro Problems In General Physics Por Wolkenstein Mir Publishers Acceleration Units Of Measurement
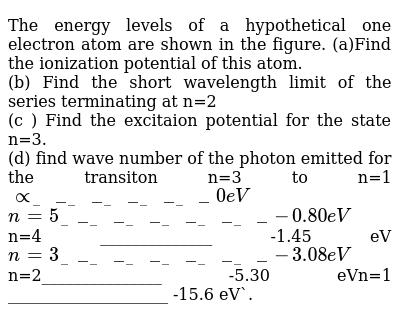



21 The Ionization Potentials Of Li And K Are 5 4 And 4 3 Ev Respe



Mrskubacki Weebly Com Uploads 8 7 3 0 Chapter 7 Practice Quiz Pdf




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is




Pet 1st Year Mains 15 07 19 Pdf Projectiles Line Geometry



Http Www2 Chem Uic Edu Tak Chem Solution set 8 Pdf




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is




A Which Has The Larger Third Ionisation Energy Be Or N B Which Has The Larger Fourth Ionisation Energy Ga Or Ge




Intro To Chem Exam 2 Flashcards Quizlet




Pet 1st Year Mains 15 07 19 Pdf Projectiles Line Geometry




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is




Periodic Table Periodic Table Ion




N S And Redox Or Not Stala Clement With Z 114 116 1187 03 Anywer The Following




B A 32r 26 Incorrect Order Of First Ionization Energy Is D G Al C Bi



Http Www2 Chem Uic Edu Tak Chem Solution set 8 Pdf



Http Www2 Chem Uic Edu Tak Chem Solution set 8 Pdf




4 Orbital With Quantum Number N 2 1 1 And M 1 Can Be Represented By 1 2px Or 2py 2 2py 3 3p2 4 2




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is




U1 Mcq Flashcards Quizlet




Periodic Table Periodic Table Ion




Qm 3 Flashcards Quizlet




Periodic Table Periodic Table Ion




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is




Pet 1st Year Mains 15 07 19 Pdf Projectiles Line Geometry



Http Www2 Chem Uic Edu Tak Chem Solution set 8 Pdf




The Correct Order Of First Ionisation Enthalpies Of The Following Elements Is



Http Www Chemmybear Com Groves Apch07 Quantumans Pdf




Chapter 14 Review Flashcards Quizlet




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is




Element With The Electronic Configuration Given Below Belong To Which Group In The Periodic Table 1s2 2s22p 3s23p63d10 4s24p64d10 5525p3 2 5th 1 3rd 3 15th 4 17th




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is




A Lithium Nucleus Has Mass 5 1e 27 Kg If Its Speed Chegg Com




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 E



Http Www Fiitjeehyderabad Com Documents Sfd Pet 1st year Mains 15 07 19 Pdf




Solved Ultiple Choice Choose The One Alternative That Be Chegg Com




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is




Nos Ordes Of Jonn Enthalpy Ne Mg Al Pisec




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is




21 The Ionization Potentials Of Li And K Are 5 4 And 4 3 Ev Respe




A Which Has The Larger Third Ionisation Energy Be Or N B Which Has The Larger Fourth Ionisation Energy Ga Or Ge




Me Cooh 46 What Is The Full Name Of The Following Compound Ch3 Hoh Hcl Ch3 A 2r 3r 3 Chloro 2 Pentanol B 2r 3s 3 Chloro 2 Pentanol C 2s 3r 3 Chloro 2 Pentanol D 2s 3s 3 Chloro 2 Pentanol 47 Which Type Of Symmetry Is Present In




Livro Problems In General Physics Por Wolkenstein Mir Publishers Acceleration Units Of Measurement



1



Http Www2 Chem Uic Edu Tak Chem Solution set 8 Pdf




Calculate The Number Of Atoms In A 84 Moles Of Li B 84 U Of Li C 84g Of Li




Periodic Table Periodic Table Ion




Chem Flashcards Quizlet



Http Www2 Chem Uic Edu Tak Chem Solution set 8 Pdf



1




Unit 5 Review Flashcards Quizlet




Periodic Table Periodic Table Ion




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is




Solved The Li Atom Has A Nucleus With A 3e Positive Charge Whic Chegg Com




Structure Of Atoni 15 199 If Ie Of He Atom Is 24 5 Ev Then Its Ie Will Be 1 24 5 Ev 2 54 4 Ev 3 108 8 Ev 4 100 Ev 2




Calculate The Number Of Atoms In A 84 Moles Of Li B 84 U Of Li C 84g Of Li




Chapter 14 Review Flashcards Quizlet




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is




The I 1 Values Of Li Be And C Are 5 4 Ev Atom 9 32 Ev Atom And 11 26 Ev Atom The I 1 Value Of Boron Is




Chapter 14 Review Flashcards Quizlet




2 Which Is Correct Order For Ii I E 1 F N O C 2 F N




Ellelgies I Iii Sc Lee Peluu 5 Arrange The Elements Of Second And Third Period Increasing




Determine The Empirical Formula Of An Oxide Otron Which By Mass 10 What Is The Difference Between A Quantum And A Photon 11 Among The Second Period Elements The Actual Ionization Enthalpies




Pet 1st Year Mains 15 07 19 Pdf Projectiles Line Geometry




Livro Problems In General Physics Por Wolkenstein Mir Publishers Acceleration Units Of Measurement




D There Can Be More Than One Sigma Bond Between Two Atoms Which Of The Following Overlaps Is Are Incorrect Assuming X Axis To Be The Internuclear Axis A 2py 2p B



Http Www2 Chem Uic Edu Tak Chem Solution set 8 Pdf




Final Ch 12 Flashcards Quizlet



0 件のコメント:
コメントを投稿